Submit INDs in Days, Not Months
From static documents to living submissions
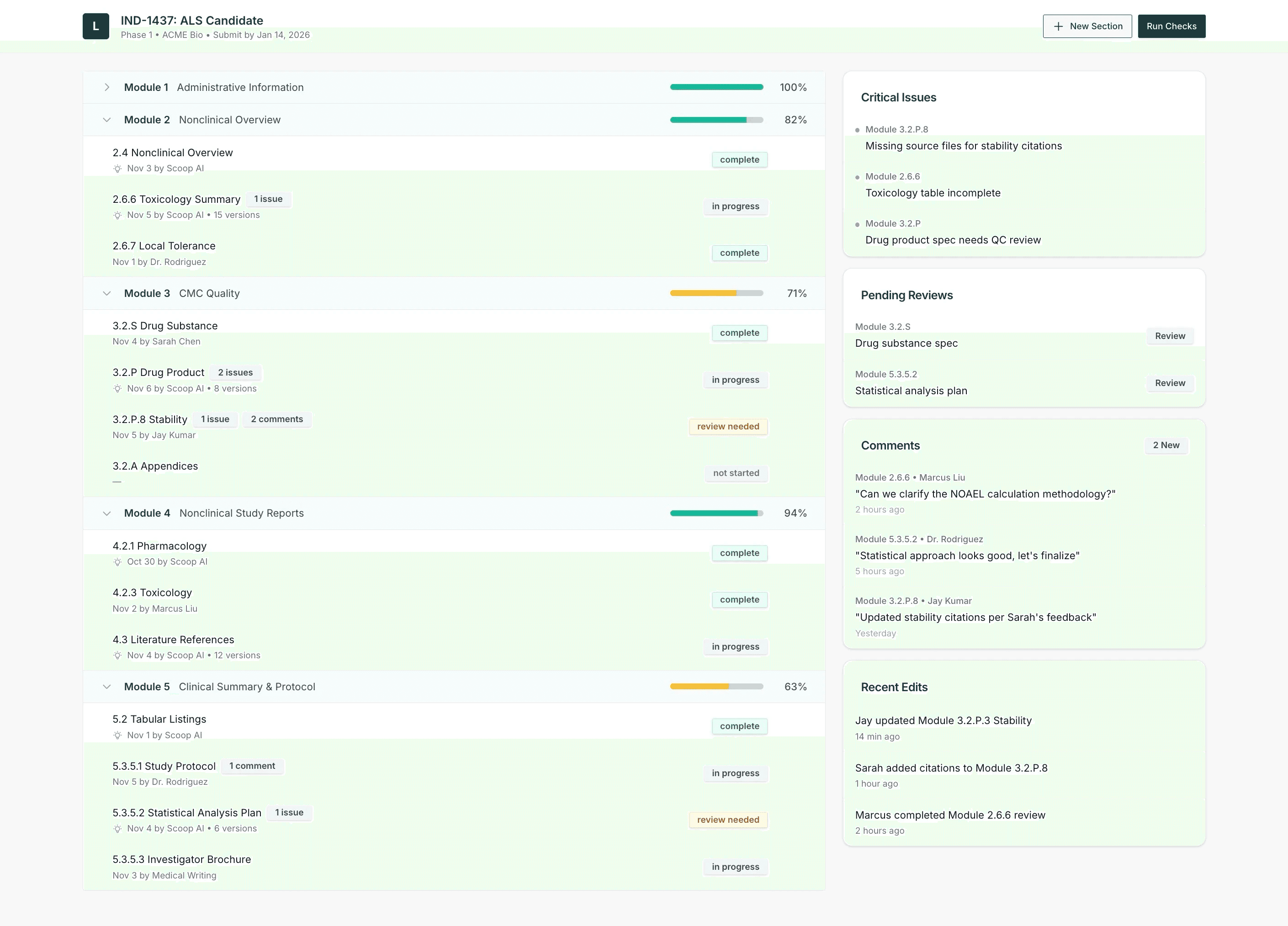
Scoop converts your study and manufacturing data into dynamic regulatory documents that stay accurate as your science advances. Our AI continuously reconciles updates, so your filings never fall out of sync with your latest results.
How It Works

Ingest
Upload scientific reports, CMC packages, and supporting documents. We classify and map the content to IND structure.
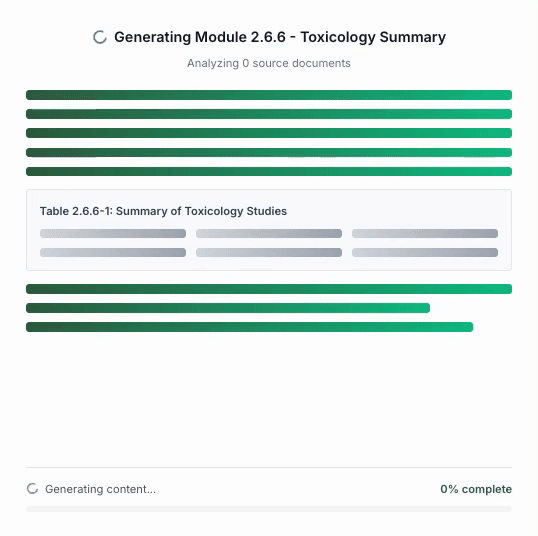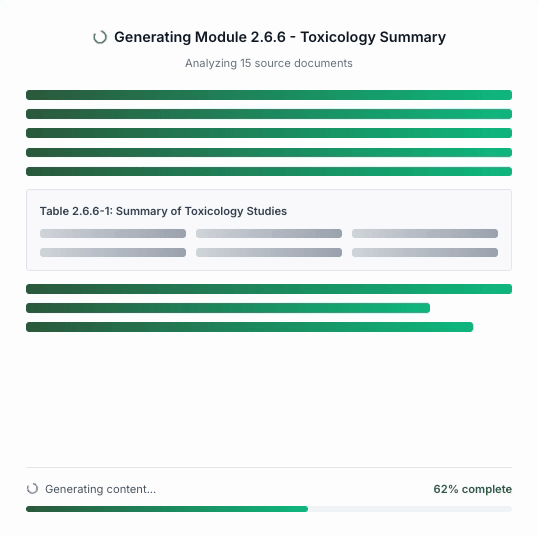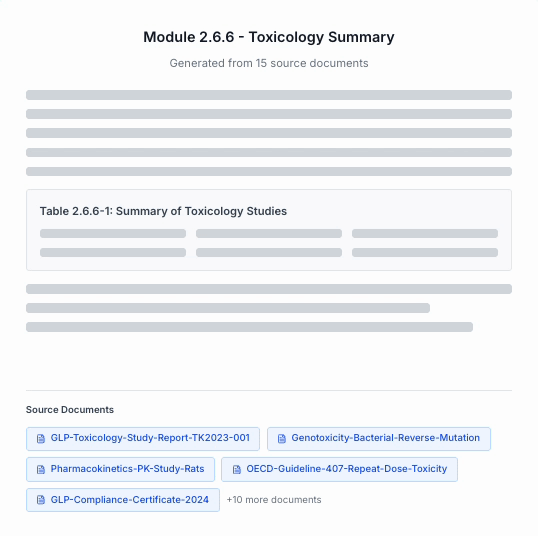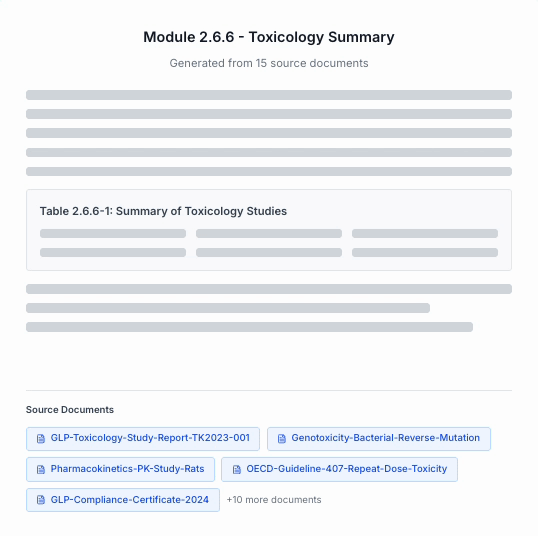
Draft
Generate submission-ready narratives with direct traceability back to every source file.
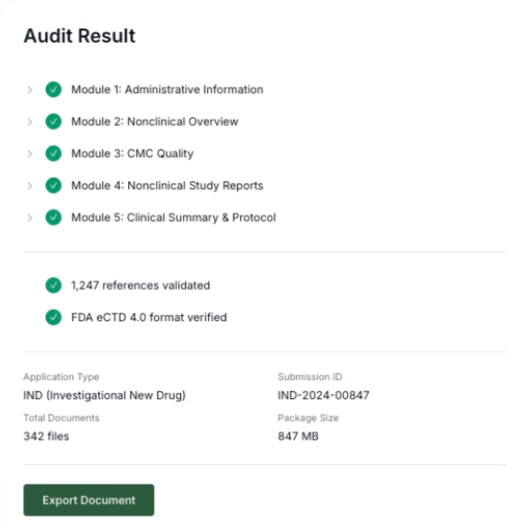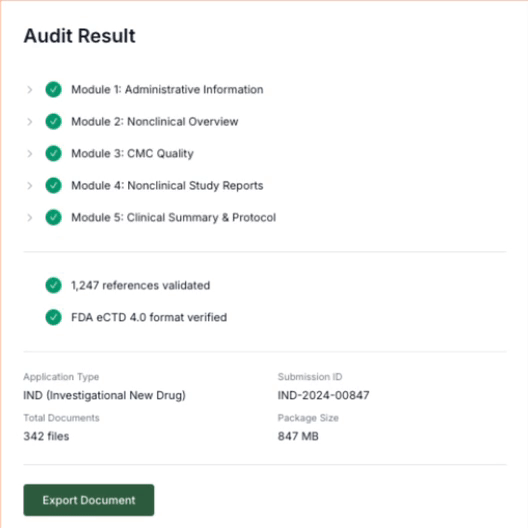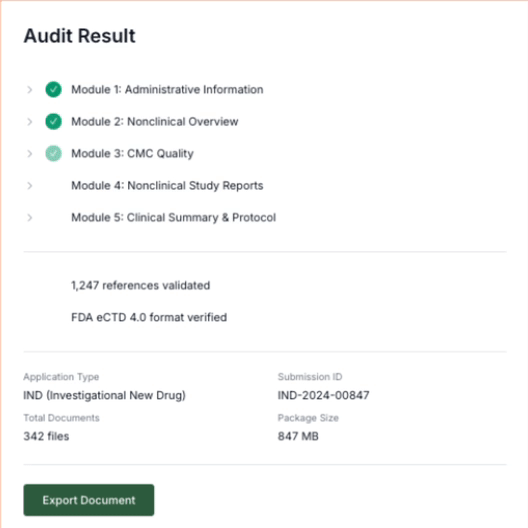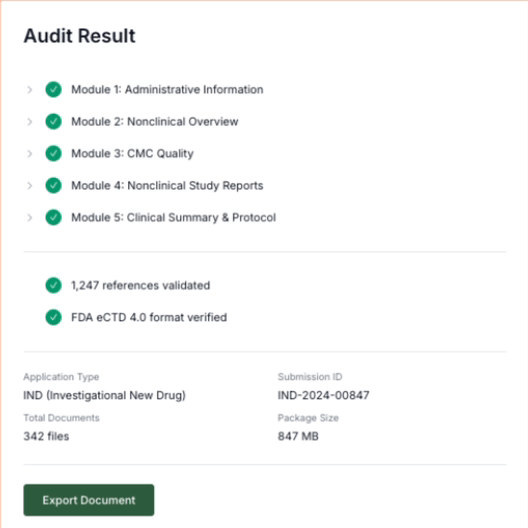
Detect Issues
The platform flags conflicting values and outdated language across sections.

Maintain
When new studies complete or manufacturing changes occur, we highlight affected sections and regenerate updated text.
The Scoop Difference
Continuously Evolving
Built for ongoing regulatory maintenance, not just a first draft.
Citation Visibility
Full traceability from every sentence to its source document.
Easy Reviews
Automatic inconsistency detection across modules.
Purpose Built
Output reads like regulatory writing, not generic summaries.
Who We Support
Regulatory Affairs Teams
Automate module drafting, formatting, and cross-referencing so you can focus on strategy, not paperwork.

CROs and Consultants
Power your clients’ submissions with AI-driven document generation that scales without adding headcount.

Modernize Your Regulatory Workflow
Questions & Answers
Learn more about what Scoop has to offer.